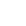Health Canada has issued a recall notice for the opioid drug, M-Eslon.
M-Eslon, or morphine sulfate, is used for long-term pain management “when pain is severe enough to require daily, around-the-clock painkillers and other treatment options are not able to treat the pain.”
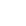
According to the recall, bottles of the drug labelled as 30 mg extended-release capsules may actually contain 60 mg.
Anyone with the drug is urged to check their medication and its packaging. If you are unsure, take it to your pharmacy.
Health Canada says the 60 mg capsule will have the number 60 and “M-ELSON” printed on it. It will also have an orange, opaque cap and a clear bottom. A photo of the two different capsules is shown below.

The incorrect dosage puts people at risk over overdose and serious health risks.
If you or someone else is experiencing an overdose, call 911 immediately.
Symptoms of morphine overdose may include: confusion, dizziness, drowsiness, difficulties waking up, small (pinpoint) pupils, slow heartbeat, slow and difficult breathing, cold and clammy skin, fainting, seizure, coma and death.
“Health Canada is monitoring the company's recall and investigation, including its implementation of corrective and preventive actions to stop this issue from reoccurring,” Health Canada added.